The Coulomb’s law was proposed by Charles Coulomb who was a French engineer and physicist. He was born on 14 June 1736 and died on 23 August 1806(70 years). He became famous due to his law called Coulomb’s law.
Before going towards coulomb’s law we must know some important terms which are related to this law.
Electric charge
The property of fundamental particle either to attract or repel each other is called electric charge. There are two types of electric charge.
- Positive charge (charge on proton)
- Negative charge (charge on the electron)
A particle having no charge is neutral and is called neutron.
Like charges repel each other while unlike charges attract each other.
The SI unit of charge is ‘Coulomb’.
Electric force
The force which holds positive and negative charges to make up atoms or molecules is called electric force.
Point charge
A charge with a very small size as compared to the distance from any other charge is called point charge. It is denoted by q.
Coulomb’s Law Rules & Explanations
Statement:
According to Coulomb’s law “the force of attraction or repulsion between two point charges is directly proportional to product of magnitude of charges and inversely proportional to the square of distance between them.”
Charles Coulomb made the first attempt to measure the magnitude of this force between the charges in 1784.
Explanation: Consider two point charges q1 and q2 and they are placed at a distance “r” from each other. So according to Coulomb’s law, the magnitude of this force is

F ∝q1 q2…………… (1)
Fα1 / r2……………… (2)
Combining equation 1 and 2;
F=k q1q2/r2
Where k=constant of proportionality which is also called electrostatic constant
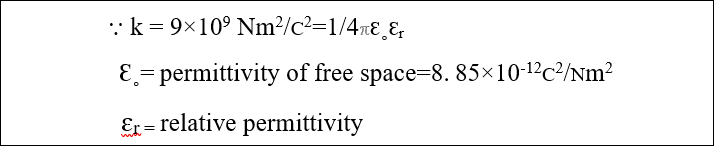
NEWTON’S THIRD LAW OF MOTION;
Coulomb’s law is in accordance with Newton’s third law of motion.it means that if q1 exerts a force on q2 then q2 also exerts a force on q1 which is equal in magnitude but opposite in direction.
Proof;
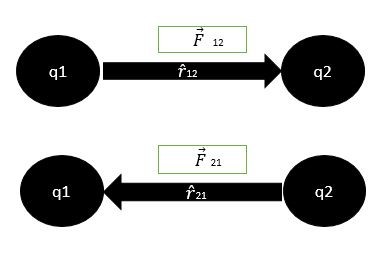
Let
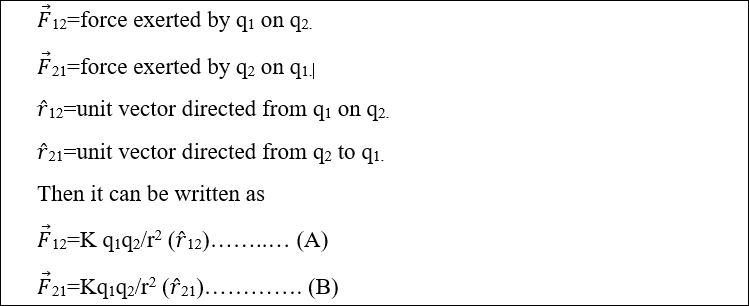
As the forces are opposite in direction so (r ̂ 12) = – ( r ̂21). The negative sign shows that their directions are opposite.so equation (A) becomes

So proved that Coulomb’s law follows Newton’s third law of motion.
Effect of insulating medium on coulomb’s force
If an insulating medium which is called dielectric medium is placed between the charges then the magnitude of Coulomb’s force decreases. The factor by which it decreases is called relative permittivity (ɛr).let
- F’=force when medium b/w charges are dielectric.
- F=force when medium b/w charges are free space.
- Thus the force in the presence of dielectric medium can be expressed as
- F’=1/4πɛ˳ɛr q1q2/r2
- F’=1/ɛr [1/4πɛ˳ q1q2/r2] ⸪1/4πɛ˳ q1q2/r2=F
- F’=1/ɛr F
- ɛr =F/F’ so
Relative permittivity
The ratio of force when medium b/w the charges is free space to the force when medium b/w them is dielectric is called relative permittivity.
Every material has its own specific ɛr value. For example;
Material ɛr
- Air 1.0006
- Vacuum 1
- Glass 4.8 ˗10
- Rubber 2.94
In this way, Charles Coulomb calculated the magnitude of force b/w two electrostatic charges. Electrostatic charges mean charges at rest.

